1. Market Overview
Regenerative medicine and cell medicine are areas of considerable potential growth. Cell medicine (sometime included in a broader definition of “regenerative medicine”) is a state-of-the-art technology that provides treatment using patients’ own (or other people’s) cells. The field of cell medicine can broadly be divided into two areas: regenerative medicine that aims to re-grow body parts such as cultured skin or cartilage, and cell transplant medicine that treats cancer or congenital diseases. Immune-cell therapy is one of the few cell medicine areas that have gone beyond the R&D stage to actual clinical use.
 Immune-cell therapy’s potential has been growing in recent years due to synergistic effects when combined with three conventional treatments (see “Substitutes”). This could lead to Immune-cell therapy developing as a baseline therapy for those conventional treatments. Minimal adverse effects associated with Immune-cell therapy could help maintain patients’ quality of life (QOL) and quality-adjusted life year (QALY). Immune-cell therapy’s potential has been growing in recent years due to synergistic effects when combined with three conventional treatments (see “Substitutes”). This could lead to Immune-cell therapy developing as a baseline therapy for those conventional treatments. Minimal adverse effects associated with Immune-cell therapy could help maintain patients’ quality of life (QOL) and quality-adjusted life year (QALY).
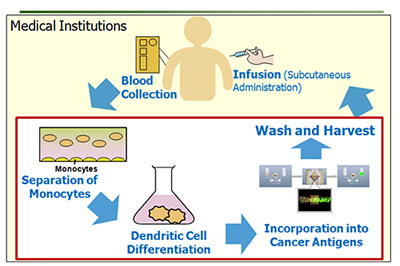
In Immune-cell therapy, the patient’s immune cells (e.g. lymphocytes) are collected, cultured/processed, and then returned to the patient’s body. The process allows suppressing the growth of cancer cells by artificially magnifying the power of immune cells.
2. Immune-cell therapy is Substitute or Add-on to Conventional Cancer Therapies Methods
The three main conventional cancer therapy methods are surgery, radiotherapy, and chemotherapy. These therapies can be administered in combination, and according to the company, it is also possible to improve therapeutic results by combining these methods with Immune-cell therapy.
Method
|
Mode
|
Characteristics
|
Target Cancers
|
Problems in Treatment
|
| Surgery |
Local |
Possibly curative for locally limited cancer |
Early-stage solid tumors |
Risk of recurrent cancer in cases where cancer cells were left unnoticed in an operation |
| Radiotherapy |
Local |
Effective against inoperable cancers due to their location |
Head and neck cancer, uterine cancer, prostate cancer, etc. |
Limited dosages of X-rays, heavy particle ion beams, etc., possible damage on normal cells |
| Chemotherapy |
Systemic |
Excellent temporary cytoreductive effects |
Almost all types of advanced cancer |
Short-term efficacy, significant adverse effects |
| Immune-cell therapy |
Systemic |
Long-term efficacy when actually effective, possibly effective against micro cancers |
Almost all types of advanced cancer |
Not delivering immediate cytoreductive effects |
3. Efficacy of Immunotherapy
- The study was carried out on cancer patients who received immune-cell therapy at the Seta Clinic Group and Allied Medical Institutions. In 5,460 cases of patients who underwent Immune-cell therapy at least six times (one course of treatment) at the Seta Clinic Group and Allied Medical Institutions from April 1999 to March 2009, the study included 1,198 cases of the primary tumor in lung, stomach, colon, liver, pancreas, breast, uterus, and ovary.
- The evaluation of the efficacy on Immune-cell therapy was conducted referring to “new guidelines to evaluation of the response to treatment in solid tumors” (which is called RECIST guidelines; internationally recognized guidelines for the evaluation of the effectiveness of medical treatment) and consisted of determining tumor reduction via diagnostic imaging. The study was conducted on 1,198 patients out of 5,460 patients who met the following criteria for determining comparative change in lesions via diagnostic imaging:
- Imaging before treatment: Images taken during a period from 60 days before the start of treatment until before the beginning of the second administration of treatment
- Imaging after treatment: Images taken during a period from the administration of the fifth treatment within 30 days after the administration of the sixth treatment
| |
Complete Responses |
Partial Responses |
Long Stable Disease |
Stable Disease |
Progressive Disease |
Total |
Response Rate |
Efficacy Rate |
Disease Control Rate |
| Lung Cancer |
2 |
57 |
43 |
125 |
135 |
362 |
16.3% |
28.2% |
62.7% |
| Stomach Cancer |
1 |
11 |
13 |
30 |
71 |
126 |
9.5% |
19.8% |
43.7% |
| Colorectal Cancer |
2 |
18 |
28 |
48 |
93 |
189 |
10.6% |
25.4% |
50.8% |
| Liver Cancer |
3 |
11 |
13 |
33 |
56 |
116 |
12.1% |
23.3% |
51.7% |
| Pancreatic Cancer |
1 |
14 |
20 |
37 |
62 |
134 |
11.2% |
26.1% |
53.7% |
| Breast Cancer |
2 |
11 |
11 |
30 |
56 |
110 |
11.8% |
21.8% |
49.1% |
| Uterus Cancer |
3 |
6 |
7 |
21 |
38 |
75 |
12.0% |
21.3% |
49.3% |
| Ovary Cancer |
3 |
15 |
10 |
18 |
40 |
86 |
20.9% |
32.6% |
53.5% |
| Total |
17 |
143 |
145 |
342 |
551 |
1,198 |
13.4% |
25.5% |
54.0% |
 |
Response Rate
= (Complete Responses + Partial Responses)/ No. of cases
Efficacy Rate
= (Complete Responses + Partial Responses + Long Stable Disease)/ No. of cases
Disease Control Rate
= (Complete Responses + Partial Responses + Long Stable Disease + Stable Disease)/ No. of cases
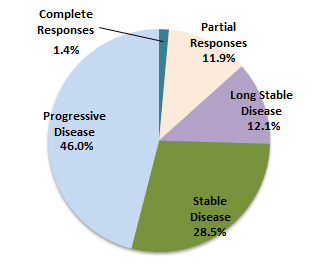
Conclusion: Out of the 1198 patients, 17 patients were complete response, 143 patients were partial response, and 145 patients were long stable disease. It was judged that immunotherapy was effective on these 305 patients.
|
(Reference: new guidelines to evaluation the response to treatment in solid tumors)
The evaluation of efficacy on the immunotherapy were conducted in accordance with the following RECIST guidelines
| Complete Response (CR): |
Disappearance of all target lesions. Any pathological lymph nodes (whether target or non-target) must have reduction in short axis to <10 mm. |
| Partial Response (PR): |
At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. |
| Progressive Disease (PD): |
At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. (Note: the appearance of one or more new lesions is also considered progression). |
| Stable Disease (SD): |
Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameters while on study |
Among those considered stable diseases, cases where the standard below applies are in the following category:
| Long Stable Disease |
Patients who have been stable for six or more months. |
|